This simple experiment is a great way to explore very basic chemistry with kids. You will probably have everything you need in your cupboards at home, but just in case you don’t – I will provide a shopping list for you below.
As I have mentioned before, my youngest is a very hands on learner and my oldest always likes the look of what we are doing so he joins in the fun too. I have found that the more messy the activity, the more engaging it is.
My kids had explained to me that they had made density towers at a recent STEM activity workshop they went to so we discussed the fact the some liquids are immiscible (dictionary definition: (of liquids) not forming a homogeneous mixture when mixed). This lead us on to our experiment.
Items needed:
- Glass Jars
- Baby Oil*
- Liquid Watercolours*
- Pipette Droppers*
- Water
- Containers for mixing water solution
We used large coffee jars and poured a whole bottle of baby oil into each one. We then made up six bowls of different coloured water by adding a few drops of colour to each bowl.
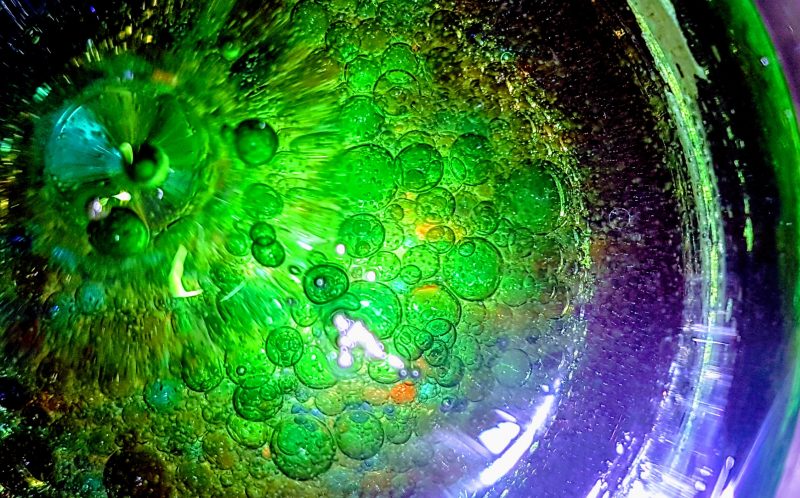
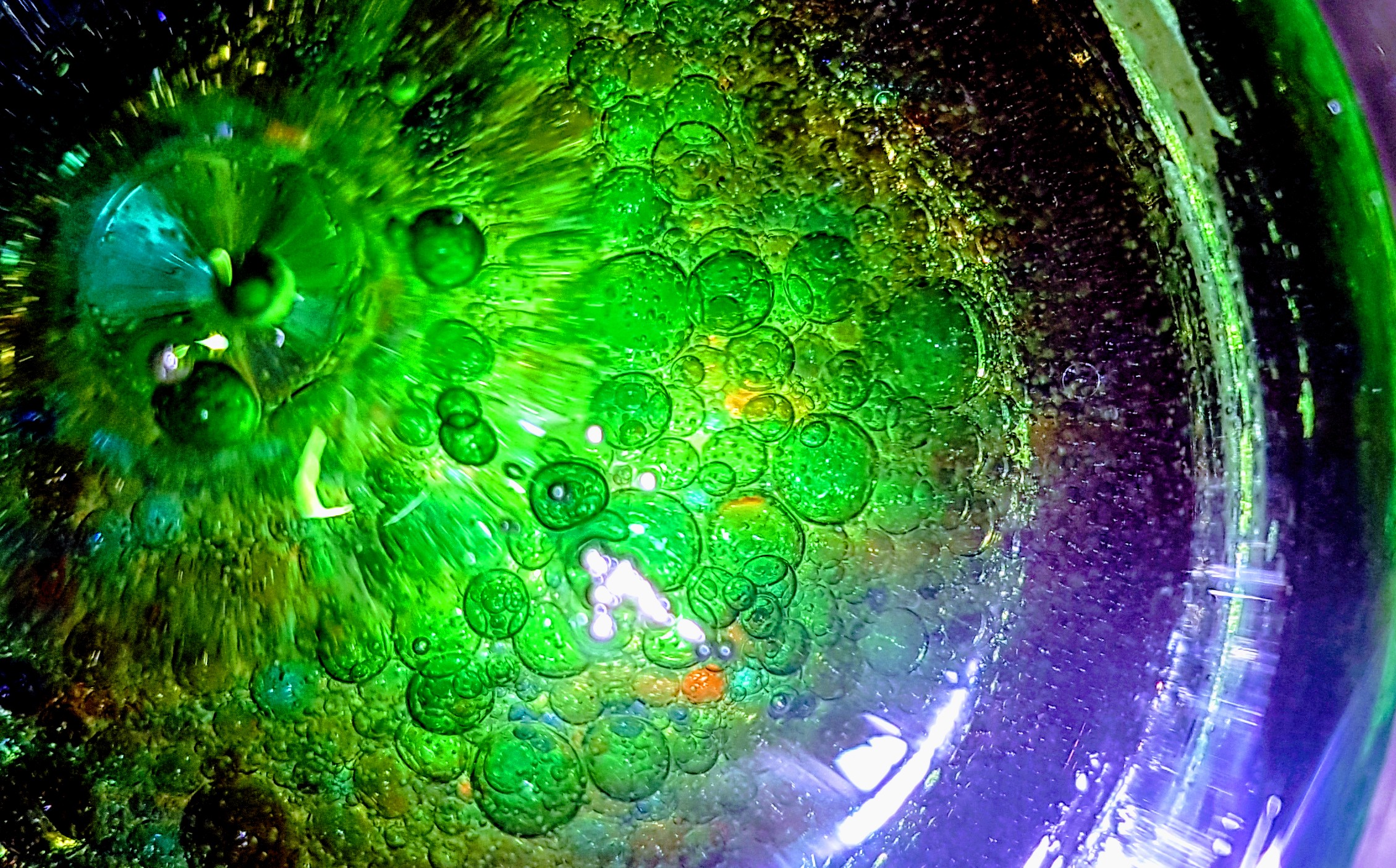
Using our pipette droppers we gradually added colour to the oil in the jars and watched what happened. The kids did some great colour combinations and we got some fantastic pictures.
So to understand that some liquids are immiscible we mixed the solutions around and each time they settled down, the liquids separated again. So oil molecules are only attracted to oil molecules and the same for water molecules. This made it easier to understand.
Secondly we learned that water has a higher density than oil and that is why the coloured solution sank to the bottom. Oil molecules are larger and therefore do not pack as tightly and take up more space per unit than water.
I hope you enjoy this simple experiment. Thanks again for stopping by.